Prominent research in Alzheimer’s disease going back for decades has been found to be fraudulent, which has effects. Alzheimer’s disease has been a cause of death with an increasing age-adjusted death rate, and it’s one of the very few causes of death with a rate that’s higher for females than males (at all ages). How has this come about? And what should be done?
Episode Links
Dan Elton from More is Different
When "weak links" in science matter -- high profile fraud in Alzheimer's disease research
Oct 20, 2024
About a year ago I wrote a post trying to gauge how much of the peer-reviewed literature in non-predatory peer-reviewed journals has deliberate fraud. A number that often comes up is 2%. However, according to forensic metascience expert James Heathers, that number is flawed and out of date. According to a recent review article by Heathers, the true number is likely around 14%.
I find many people are either not aware of this issue, or they acknowledge the issue but wave it away as not important.
An example is someone who I met recently who works at the National Science Foundation. From their vantage point, fraud is mostly being committed in China, not in the USA, which they described as having far superior systems for grant dispersal and oversight. This is largely true, but it doesn’t mean fraud from the US is not an important issue.
One example of retracted article
Original article
APP binds DR6 to trigger axon pruning and neuron death via distinct caspases
Nature, 2009 Feb 19;457(7232):981-9. doi: 10.1038/nature07767.
Abstract
Naturally occurring axonal pruning and neuronal cell death help to sculpt neuronal connections during development, but their mechanistic basis remains poorly understood. Here we report that beta-amyloid precursor protein (APP) and death receptor 6 (DR6, also known as TNFRSF21) activate a widespread caspase-dependent self-destruction program. DR6 is broadly expressed by developing neurons, and is required for normal cell body death and axonal pruning both in vivo and after trophic-factor deprivation in vitro. Unlike neuronal cell body apoptosis, which requires caspase 3, we show that axonal degeneration requires caspase 6, which is activated in a punctate pattern that parallels the pattern of axonal fragmentation. DR6 is activated locally by an inactive surface ligand(s) that is released in an active form after trophic-factor deprivation, and we identify APP as a DR6 ligand. Trophic-factor deprivation triggers the shedding of surface APP in a beta-secretase (BACE)-dependent manner. Loss- and gain-of-function studies support a model in which a cleaved amino-terminal fragment of APP (N-APP) binds DR6 and triggers degeneration. Genetic support is provided by a common neuromuscular junction phenotype in mutant mice. Our results indicate that APP and DR6 are components of a neuronal self-destruction pathway, and suggest that an extracellular fragment of APP, acting via DR6 and caspase 6, contributes to Alzheimer's disease.
Retraction notice
Retraction Note: APP binds DR6 to trigger axon pruning and neuron death via distinct caspases
The authors have retracted this article1. Our subsequent work confirmed aspects of the article, notably that DR6 and APP interact and function in a genetic pathway involving caspases to control axon pruning and neuron death2,3. However, our later research also showed that certain conclusions reached in the article were incorrect, notably the role of caspase-3, the necessity for beta-secretase enzyme activity for APP-DR6 binding, and the model for the APP-DR6 interaction2,3.
More recently, the following anomalies were identified:
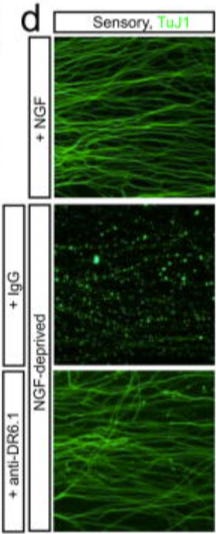
Figure 1d: the NGF-deprived +IgG panel appears to be identical to the NGF-deprived, 24h + Control IgG panel of Figure 5e.
Supplementary information Figure 9c: the NGF-deprived + Bax inhibitor control panel appears to be identical to the + anti-NGF control panel of Supplementary information Figure 17c.
Supplementary information Figure 6d: the fourth beta-Actin blot for Casp-3 siRNA appears to be identical to the first beta-Actin blot for Casp-6 siRNA.
Certain biostatistical calculations underlying some figures contained errors.
We believe that these additional anomalies do not affect the conclusions presented in the affected figures. However, given the lack of original data for several of these figures due to the age of the paper, and since our subsequent research showed that certain specific claims in the original article were not correct and we reported a correction for those claims elsewhere2,3, we consider that the appropriate course of action is to retract the article. All the authors agree with this retraction.
Figure 1d:
Figure 5e:
Ben Landau-Taylor from Palladium Magazine
August 2, 2024
In 2006, Sylvain Lesné and seven coauthors published a paper on Alzheimer’s disease, “A specific amyloid-beta protein assembly in the brain impairs memory,” in Nature, the world’s most prestigious scientific journal. This was a major paper in the development of the “amyloid hypothesis,” a proposed mechanism for how Alzheimer’s disease afflicts its victims. About 50 million people suffer from Alzheimer’s disease, more than the entire population of California, making it the world’s most common cause of dementia. This population will grow as the world’s average population gets older. There is no effective treatment for Alzheimer’s disease, and its pathology is poorly understood. Any progress in understanding this disease represents a massive humanitarian victory. Encouraged by this paper and other promising studies, funding and talent poured into investigating the amyloid hypothesis. By 2022, such research had received over $1 billion in government funds.
That year, neuroscientist Matthew Schrag discovered doctored images in this and many of Lesné’s other papers, including others purporting to provide evidence for the amyloid hypothesis. These images had been manually edited and cropped together to falsely show support for the papers’ hypotheses. Notably, these frauds all made it through the formalized “peer review” processes of Nature and six other academic journals undetected, before eventually being uncovered by unrelated channels.
Schrag’s investigation that uncovered the fraudulent papers began as a tangent from his work uncovering doctored images used in studies supporting simufilam, an experimental drug for Alzheimer’s disease. The suspicion would prove vindicated when in June 2024 Hoau-Yan Wang, a paid adviser to simufilam’s developer, was indicted by a federal grand jury for fabricating data and images in simufilam studies for which he obtained $16 million in National Institutes of Health (NIH) grants, following a 2021 petition to the Food and Drug Administration, a method of reporting research fraud which is highly unusual if not unique.
Theo Baker in The Stanford Daily
Feb 17, 2023
In 2009, Marc Tessier-Lavigne, then a top executive at the biotechnology company Genentech, was the primary author of a scientific paper published in the prestigious journal Nature that claimed to have found the potential cause for brain degeneration in Alzheimer’s patients. “Because of this research,” read Genentech’s annual letter to shareholders, “we are working to develop both antibodies and small molecules that may attack Alzheimer’s from a novel entry point and help the millions of people who currently suffer from this devastating disease.”
But after several unsuccessful attempts to reproduce the research, the paper became the subject of an internal review by Genentech’s Research Review Committee (RRC), according to four high-level Genentech employees at the time; two were senior scientists and two were scientists who also served as executives. Three spoke on the condition of anonymity because of the sensitivity of the allegations and non-disclosure agreements. The scientists, one of whom was an executive who sat on the review committee and all of whom were informed of the review’s findings at the time due to their stature at the company, said that the inquiry discovered falsification of data in the research, and that Tessier-Lavigne kept the finding from becoming public.
Tessier-Lavigne denies both allegations. Genentech said in a statement that “as part of our diligence related to these allegations, we reviewed the records from that November 2011 RRC meeting and saw no allegations of fraud or wrongdoing.” The company acknowledged that “given that these events happened many years ago … our current records may not be complete.”
After the review, which began in 2011, Genentech canceled research based on the paper’s findings. Till Maurer, a senior scientist at the company from 2009-2018 who said he was assigned to develop drugs based on the 2009 paper, told The Daily that his superior informed him that, in Maurer’s words, “the project is being canceled and it’s because they found falsified data.”
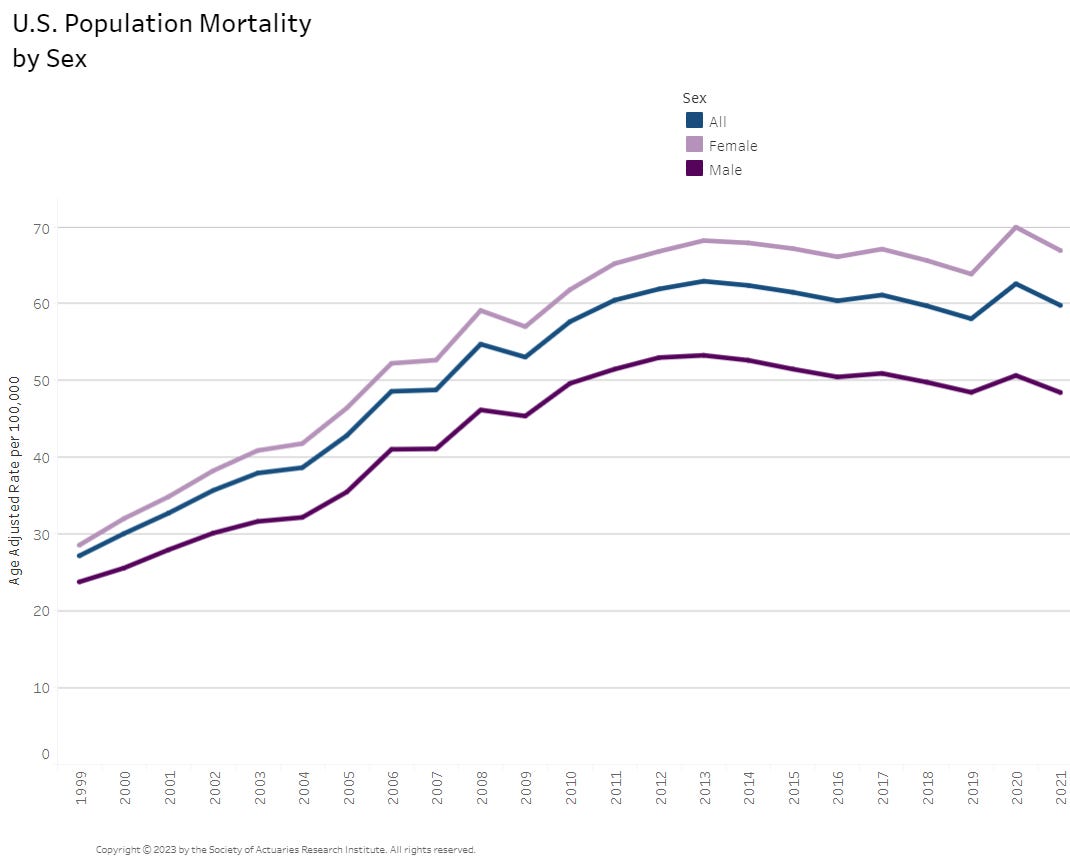
Alzheimer’s Mortality in the U.S. via the Society of Actuaries
U.S. Population Mortality Observations – Updated with 2021 Experience
Tableau dashboard:


















Share this post